Emerging as promising, safe treatment option for Covid patients in US
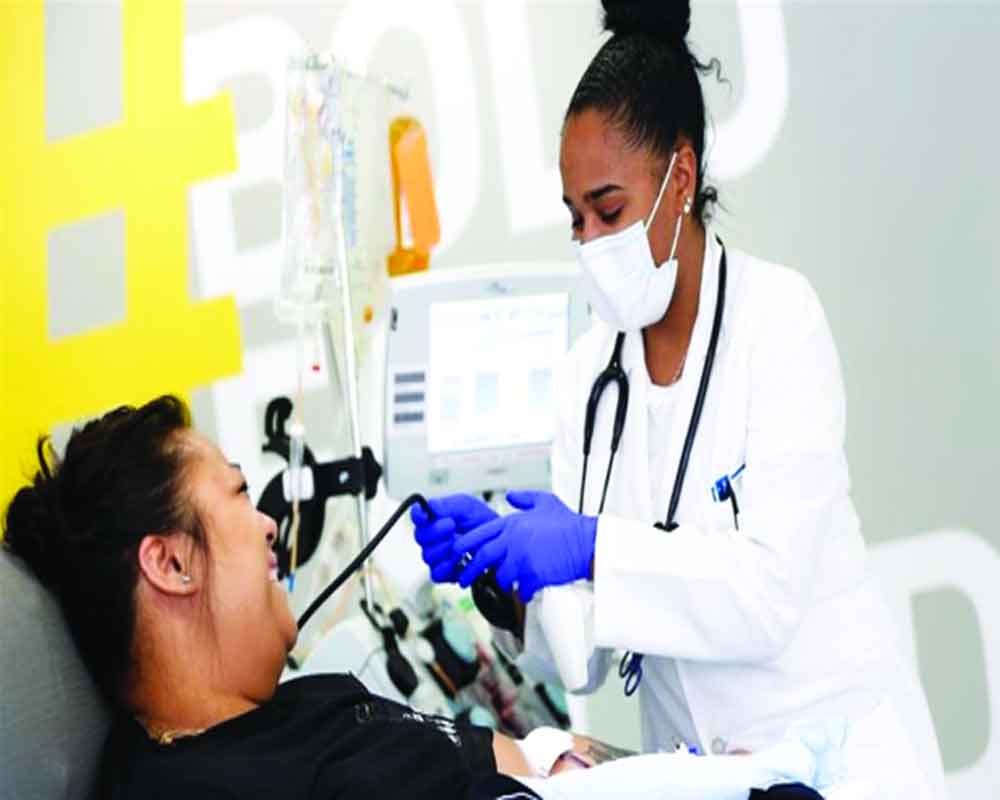
Convalescent plasma therapy is emerging as one of the most promising and safe treatment options for patients with severe Covid-19 symptoms, a new trial in the US has found.
The therapy involves taking anti-bodies from the blood of a person who has recovered from Covid-19 and transfusing those anti-bodies into an active coronavirus patient to help kickstart the immune system to fight the infection.
Published in The American Journal of Pathology, the study conducted by the researchers from the Houston Methodist Hospital in the US said that the results of the trial showed that 19 out of 25 patients (76 per cent) improved with the treatment and 11 were discharged from the hospital. There were no serious adverse side effects caused by the plasma transfusion.
The findings have generated hopes in the global community rattled by the virulent virus which has claimed over 3 lakh people with over 63 lakh infected cases across the world.
The clinical trials to transfuse plasma from recovered Covid-19 patients into critically ill patients started on March 28.
“While physician-scientists around the world scramble to test new drugs and treatments against the Covid-19 virus, convalescent serum therapy emerge as potentially one of the most promising strategies,” said study researcher Eric Salazar from Houston Methodist Hospital.
Patients were treated under emergency use guidelines (eIND) from the US Food and Drug Administration and then received approval from the FDA to open up the trial to more patients as an investigational new drug (IND).
According to the researchers, the therapeutic approach dates back to at least as early as 1918 when it was used to fight the Spanish Flu. Recently, the convalescent plasma therapy was used with some success during the 2003 SARS pandemic, the 2009 influenza H1N1 pandemic, and the 2015 Ebola outbreak in Africa.
Following a study early on in the Covid-19 pandemic, where a handful of critically ill patients in China showed an improvement, an interdisciplinary team of Houston Methodist physician-scientists and healthcare workers rapidly targeted the Covid-19 virus with convalescent serum therapy.
The research team also concluded that any observed complications were consistent with findings reported for Covid-19 disease progression and did not result from the plasma transfusions.
The overall findings were consistent with several other small case studies of convalescent plasma use for severe Covid-19 that has been recently reported.
Although the convalescent plasma therapy administered on the frontlines at Houston Methodist was implemented for emergency treatment, they said there is an immediate need for controlled clinical trials to determine its efficacy.
Researchers are considering a randomised controlled trial to look more closely at variables such as the timing of the transfusion after the onset of symptoms.
In India, where virus has already infected 2 lakh people and killed over 5,000, the Indian Council of Medical Research (ICMR) has approved 21 institutions for participating in a randomised controlled study to assess the safety and efficacy of convalescent plasma to limit complications associated with the virus.
The sample size of the study is 452. Once 400 patients are enrolled, no more sites will be added. The clinical trial liability insurance has been bought centrally by the ICMR, officials said.