Covid-19 vaccine makers' factsheets to help recipients understand shots' risks & benefits
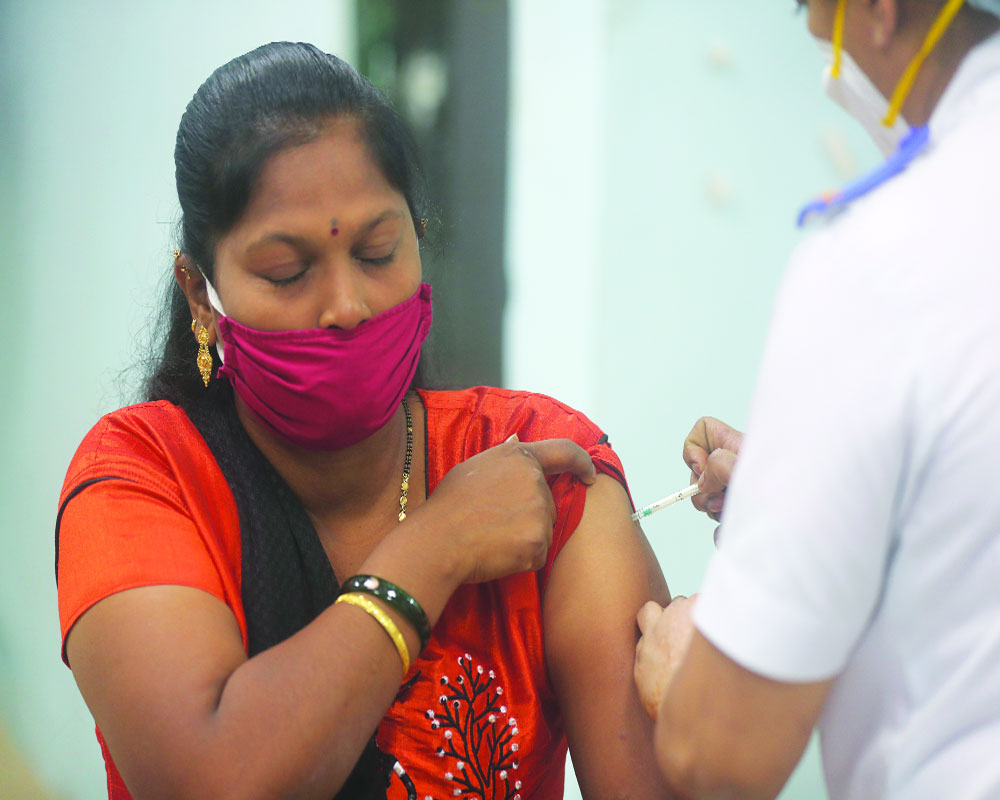
Three days after the launch of the nationwide vaccination drive and reports of several adverse events following immunisation (AEFI), Serum Institute of India (SII) and Bharat Biotech, makers of anti-Covid vaccine Covishield and Covaxin respectively, released a set of “Dos and don’ts aiming to help a recipient understand the risks and benefits of their vaccine.”
Interestingly, soon after the approval of their vaccine by the DCGI early January, both firms had questioned the efficacy of each other’s jabs.
The Bharat Biotech factsheet said that “it is advisable not to take the vaccine if a person has allergies, fever or bleeding disorder or is on a blood thinner. It also said that pregnant and breastfeeding women should also avoid taking Covaxin.”
Those who are immune-compromised or are on medicine that affects immune system, and those who have received another Covid-19 vaccine should also not get the Bharat Biotech’s medicine, said the company whose vaccine is being refused by a section of doctors like the Resident Doctors’ Association (RDA) of RML hospital in Delhi and Karnataka to name a few.
They have preferred Covishield over Covaxin.
Private practitioners like Dr Rahul Bhargava, Director (Institute of Blood Disorder and Bone Marrow Transplant) Fortis Hospital, Gurugram, too, minced no words as he doubted the efficacy of the Covaxin.
He also felt that the two companies should have released the factsheets prior to the mega vaccination drive so that people were not only aware of the health impacts of the vaccines but also whether or not they are eligible for it.
The Hyderabad-based biotechnology firm also said that the DCGI has authorised the restricted use of its vaccine under clinical trial mode.
“Individuals, who are prioritised under the public health programme of the Ministry of Health and Family welfare, will be covered under this endeavour. Informing the individuals about the offer for vaccination with Covaxin will rest with the respective Government programme officials. Those offered Covaxin at pre-specified booths will have the options to receive or reject administration of the vaccine,” the factsheet stated.
The company document further described the ingredients in the Covaxin. It contains 64g of whole-virion inactivated SARS-CoV-2 antigen (Strain: NIV-2020-770), and the other inactive ingredients such as aluminum hydroxide gel (250 µg), TLR 7/8 against (imidazoquinolinone) 15 µg, 2-phenoxyethanol 2.5 mg, and phosphate buffer saline up to 0.5 ml.
“The vaccine thus has been developed by using inactivated/killed virus along with the aforementioned chemicals,” it said, adding that Covaxin is administered as an injection into the deltoid muscle of the upper arm. It is a two-dose series given four weeks apart.
Not keen to be left behind in the race, within minutes the SII too released its factsheet, stating that people who are severely allergic to any ingredient of Covishield are advised not to take it. “Pregnant and lactating mothers should mention it to the healthcare provider before getting the jab. Also, do not forget to take the second dose!”
The SII said that one should not get the Covishield vaccine if the person had a severe allergic reaction after a previous dose of this vaccine which has “L-Histidine, L-Histidine hydrochloride monohydrate, Magnesium chloride hexahydrate, Polysorbate 80, Ethanol, Sucrose, Sodium chloride, Disodium edetate dihydrate (EDTA), water for injection,” as its ingredients.
About the possible adverse effect of the vaccine, the SII said that “some of the very common side effects of the vaccines are tenderness, pain, warmth, redness, itching, swelling or bruising where the injection is given, generally feeling unwell, chills or feeling feverish, headache or joint aches.”
However, if you feel dizzy, lack of appetite, abdominal pain, enlarged lymph nodes, excessive sweating, itchy skin or rash, then you should contact the doctor as these are not very common symptoms, the release said.
The vaccine maker also said that the vaccine recipient should also tell the healthcare provider about all the medical conditions before getting the Covishield vaccine including, “if you have ever had a severe allergic reaction (anaphylaxis) after any drug, food, any vaccine or any ingredients of Covishield vaccine”.
The recipients should also mention to the healthcare provider, if they have fever, if they have a bleeding disorder or are on a blood thinner and also if they are immuno-compromised or are on a medicine that affects your immune system, it added.
Also, when you get your dose, please discuss with your healthcare provider regarding the option of your vaccination record on a digital platform, if available. The second dose is essential which should be administered between 4 to 6 weeks after the first dose.