What does the case of the volunteer in Chennai tell us about medical trials?
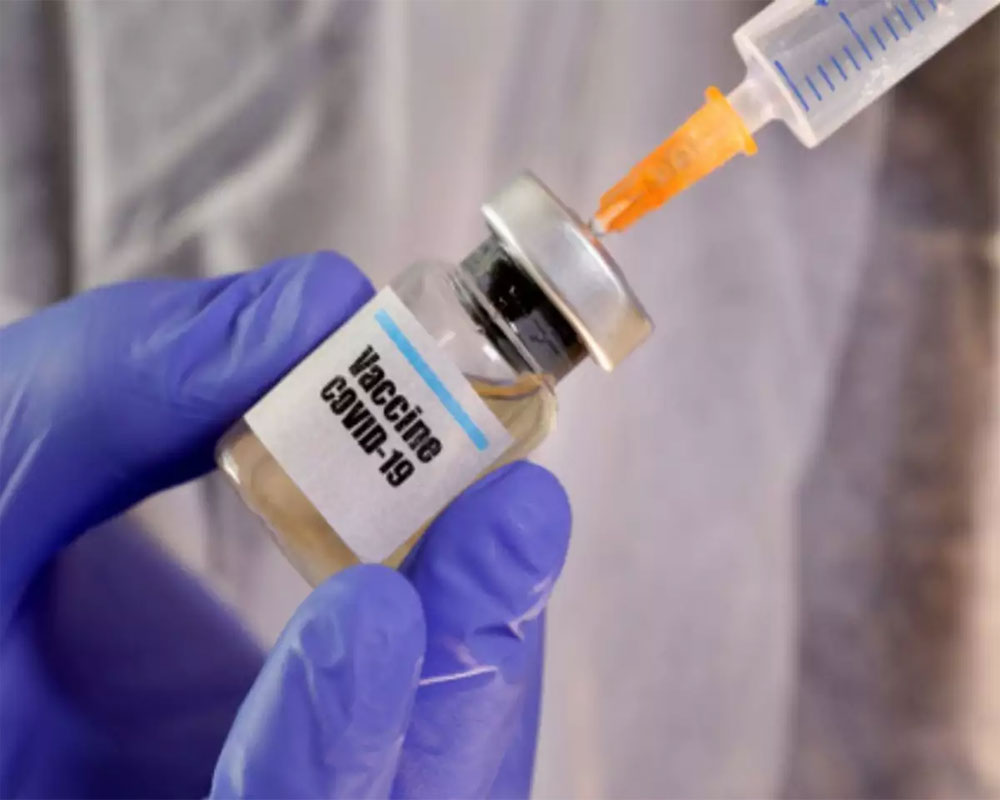
While a 40-year old man, apparently a business consultant from Chennai, has claimed that he has suffered from adverse reactions to the trial vaccine administered by the Serum Institute of India (SII) during the Phase-III clinical trials, we do not know the full details of the case. This is the vaccine developed by Anglo-Swedish pharmaceutical firm AstraZeneca and University of Oxford where SII is a partner. For one, we still do not know whether the individual was given the new vaccine or a placebo, which is why his allegations raised by his lawyers are slightly suspect. The instant countersuit by SII makes one suspect whether this was just an extortion attempt but one thing is clear, the individual involved has little chance of success in court as volunteers to clinical trials for any medication should be fully aware that they can suffer extremely adverse reactions to trial medication and those reactions could also mean death.
The extremely rushed research and development for vaccines to counter the Covid-19 virus, which has devastated the global economy and driven millions across the world back into poverty, might yet be successful but rushing clinical trials will mean that certain adverse effects and even a death or two might occur. Those who volunteer for such trials should be made fully aware of the risks and they usually are. In case this is genuine, and it well might be, the individual and his family should be compensated for his tribulations. The system of clinical trials in India should ensure that those who take upon the civic responsibility to better mankind by subjecting their bodies to such insane risks should be given adequate coverage for the risks involved. Ever so often in India, many trials are highly risky and those who volunteer, often from poorer families, are dumped and left without compensation or recourse if things go wrong. There may be more than what meets the eye in this particular case. But that should not take away from the tremendous risks and troubles that many clinical trial volunteers put themselves through. This also highlights that companies involved in clinical trials, whether they are large, global pharmaceutical firms or small research firms in India, should be more open and transparent about what the volunteers suffer. SII would do well to tell us the entire story lest this becomes a case where the anti-vaccine movement can get a foot in the door in India.